Abivax provides business and operational update
- ABTECT Phase 3 Global Clinical Trial Enrollment Progressing as Planned; Induction and Maintenance Data Readouts Expected in Q1 2025 and Q1 2026, Respectively
- Durability of Efficacy Results with No New Safety Signals Observed in Step-Down Dosing from 50 mg to 25 mg for Approximately the Third and Fifth Year of Open-Label Maintenance Treatment with Obefazimod in Ulcerative Colitis (“UC”) Patients
- Prioritizing the Goal of Expanding the Global IP Position of Obefazimod Beyond 2035 up to 2039
- Expansion of U.S. Operations Commences with Focus on Potential Future Commercialization of Obefazimod in the U.S. Inflammatory Bowel Disease (“IBD”) Market
- Multi-Pronged Strategy to Support Financial Stability through Q2 2024 (with possible extension to Q4 2024 with drawdown of all debt facilities) with Catalysts Expected into 2026 and Operational Excellence Reinforced with Strengthened Management Team and Additional Board Members
PARIS, France, September 7, 2023 – 08:00 a.m. (CEST) – Abivax SA (Euronext Paris: FR0012333284 – ABVX) (“Abivax” or the “Company”) today announced updated business and operational goals along with changes to Abivax’s overall strategy, focused on preparing Abivax for the potential commercialization of its investigational lead asset, obefazimod, in IBD.
Marc de Garidel, Chief Executive Officer of Abivax, says: “With our strategic roadmap in place, we are ready to move Abivax into its next chapter by strengthening our position within the U.S. We are working to extend patent protection for obefazimod and have brought on an experienced leadership team to help prepare obefazimod for commercialization in the U.S. Furthermore, we are in the process of executing a multi-pronged strategy to secure our future business and financial objectives.”
Didier Blondel, Chief Financial Officer of Abivax, continues: “In 2023, we have successfully executed two financing rounds in equity and debt with top-tier investors, which we believe indicates investor confidence and belief in the potential of obefazimod. Moving forward, we intend to look for opportunities to further extend our operating capital, allowing us to deliver on our clinical development goals.”
BUSINESS AND OPERATIONAL UPDATE
Strategic priorities
- Advance Obefazimod—Establish obefazimod as a potential first-line advanced therapy for the treatment of IBD. This goal is based on (i) robust Phase 2a and 2b clinical trial data in patients with moderately to severely active UC, as well as (ii) obefazimod’s novel mechanism of action that was demonstrated to enhance the expression of miR-124, a natural regulator of the inflammatory response. Initiation of the Phase 2a clinical trial in Crohn’s disease (“CD”) is expected in 2024 and evaluation of potential combination therapy opportunities in UC is ongoing.
- Optimize Opportunity in IBD in the Near Term with Phase 3 Data Beginning in 2025—Overcome limitations of currently available treatments for UC to establish obefazimod as a differentiated treatment option with the goal of providing convenient oral administration, safety, tolerability, and durable efficacy.
- Leverage Library of miR-124 Enhancers—Explore and expand development options of obefazimod in other inflammatory conditions and continue R&D work to identify additional drug candidates from Abivax’s proprietary small molecule library that includes additional miR-124 enhancers.
ABTECT Phase 3 clinical program in UC
Abivax’s focus is meeting enrollment goals of the ABTECT Phase 3 program with obefazimod for the treatment of moderately to severely active UC.
- Primary endpoint for both induction trials is clinical remission at 8 weeks; for the maintenance trial it is clinical remission at 52 weeks (which is week 44 of the maintenance trial).
- ABTECT top-line induction data readout is expected in Q1 2025; top-line maintenance trial data readout expected in Q1 2026.
Product pipeline and development milestone
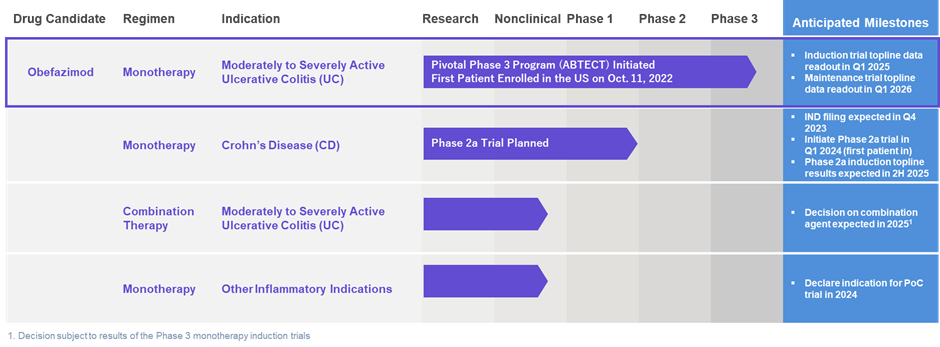
- Obefazimod in Crohn’s Disease—Based on existing supportive data, Abivax plans to advance obefazimod in moderately to severely active CD. Preparations for an expected Investigational New Drug application in Q4 2023 are ongoing. A Phase 2a trial of obefazimod in CD is expected to start recruitment in Q1 2024, with top-line induction results expected in 2H 2025.
- Obefazimod in Combination Therapy—Based on its early clinical profile, the use of obefazimod as a combination therapy for the treatment of moderately to severely active UC is expected to be explored.
- Obefazimod in Other Inflammatory Conditions—The anti-inflammatory effect of obefazimod observed during Phase 2 trials to date is encouraging and Abivax is exploring the potential of obefazimod in additional chronic inflammatory conditions. In 2024, Abivax expects to make decisions on other potential target indications.
- Compound miR-124 Library—R&D work on potential follow-on drug candidates to be selected from Abivax’s optimized compound library will continue. Pre-clinical development for the first selected follow-on drug candidate is expected to start in 2024 to further strengthen the pipeline.
Step-down dosing from 50 mg to 25 mg for approximately the third and fifth year of open-label maintenance treatment with obefazimod in UC patients
- UC patients treated with 50 mg of oral, once-daily, obefazimod completing approximately four years of treatment in the Phase 2a program and approximately two years of treatment in the Phase 2b program, if eligible (Mayo Endoscopic subscore = 0 or 1, normal or mild disease), could roll over into a follow-on, open-label, maintenance trial with a reduced dose of 25 mg.
- In an interim analysis (cut-off date of July 31, 2023) of the 71 eligible patients, 63 completed their 48-week visit, with a demonstrated disease control rate (stable or improved Modified Mayo Score) of 84% (53 of 63 patients) with the 25 mg once-daily dose of obefazimod.
- No new safety signals were detected in UC patients treated up to five years with oral, once-daily obefazimod.
- Full results expected to be submitted for presentation at upcoming medical conferences.
Strengthening of IP position
One of the two patents for obefazimod in the U.S. will be selected for Patent Term Extension (PTE) from 2035 to 2039. Potential extension of the method of use patents through PTE for obefazimod was assessed and confirmed by two globally recognized IP law firms.
Composition of matter patent or method of use patent (both granted) would extend the product patent protection until 2035 or the use patent until 2040 in the EU.
Expansion of U.S. operations and leadership team
The establishment of an Abivax U.S. presence is currently in progress.
- U.S. employee presence has been strengthened to execute commercial launch preparation activities for obefazimod.
- The addition of new executive team members with global expertise commercializing drug candidates in the immunology and IBD market.
- Abivax U.S. office currently planned to open in the Greater Boston Area in Q4 2023.
- Dual source Contract Manufacturing Organization (CMO) presence to be established in North America to complement EU CMO.
- June Lee and Troy Ignelzi have joined the Abivax Board of Directors to add to existing skills and U.S. competencies and diversity.
FINANCIAL UPDATE
- With €130M gross equity financing (€123M net proceeds) raised in February 2023 and two additional structured debt agreements (€27M net proceeds from the first tranches) signed in August 2023, Abivax has €118M cash on hand (as of August 2023, unaudited) and expects its current cash runway to finance Abivax’s operations through Q2 2024. With an additional €90M financing that can be accessed by leveraging the existing debt agreements beyond the recent draw downs (subject to certain conditions precedent being met), Abivax could extend its cash runway into Q4 2024.
- The new strategic pre-clinical and clinical initiatives as described above, as well as the expansion of Abivax’s clinical, medical and commercial capabilities, will require additional capital.
- To help ensure long-term financing and extend its current cash runway, Abivax is implementing a multi-pronged financing strategy. The final funding size and equity and debt allocation is expected to be made with the priority of funding Abivax’s strategic initiatives.
*****
Financial agenda:
- Thursday, September 21, 2023: Publication of financial statements as of June 30, 2023
- Friday, September 29, 2023: Publication and release of 2023 half year report
*****
Contacts:
Abivax Communications Regina Jehle regina.jehle@abivax.com +33 6 24 50 69 63 |
Abivax |
Public Relations France |
FORWARD-LOOKING STATEMENTS
This press release contains forward-looking statements, forecasts and estimates, including those relating to the Company’s business and financial objectives. Words such as “continue,” “could,” “expect,” “goal,” “intend,” “objective,” "will" and variations of such words and similar expressions are intended to identify forward-looking statements. Although Abivax’ management believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks, contingencies and uncertainties, many of which are difficult to predict and generally beyond the control of Abivax, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. A description of these risks, contingencies and uncertainties can be found in the documents filed by the Company with the French Autorité des Marchés Financiers pursuant to its legal obligations including its universal registration document (Document d’Enregistrement Universel). These risks, contingencies and uncertainties include among other things, the uncertainties inherent in research and development, future clinical data and analysis, decisions by regulatory authorities, such as the FDA or the EMA, regarding whether and when to approve any drug candidate, as well as their decisions regarding labelling and other matters that could affect the availability or commercial potential of such product candidates. Special consideration should be given to the potential hurdles of clinical and pharmaceutical development including further assessment by the company and regulatory agencies and IRBs/ethics committees following the assessment of preclinical, pharmacokinetic, carcinogenicity, toxicity, CMC and clinical data. Furthermore, these forward-looking statements, forecasts and estimates are only as of the date of this press release. Readers are cautioned not to place undue reliance on these forward-looking statements. Abivax disclaims any obligation to update these forward-looking statements, forecasts or estimates to reflect any subsequent changes that the Company becomes aware of, except as required by law. Information about pharmaceutical products (including products currently in development) which is included in this press release is not intended to constitute an advertisement. This press release is for information purposes only, and the information contained herein does not constitute either an offer to sell, or the solicitation of an offer to purchase or subscribe securities of the Company in any jurisdiction. Similarly, it does not give and should not be treated as giving investment advice. It has no connection with the investment objectives, financial situation or specific needs of any recipient. It should not be regarded by recipients as a substitute for exercise of their own judgement. All opinions expressed herein are subject to change without notice. The distribution of this document may be restricted by law in certain jurisdictions. Persons into whose possession this document comes are required to inform themselves about and to observe any such restrictions.
